Oli Genn-Bash explores the growing popularity of functional mushrooms, blending personal experience, research, and traditional perspectives to argue for a more holistic, respectful understanding of fungi.

in this article
- CYB003 Receives Regulatory Approval for MDD
- The Minnesota Psychedelic Medicine Task Force
- Psychedelics Beneficial for the Clergy?
- Experimental Psychedelics for Life-Threatening Diagnoses
- Psychedelics Help LGBTQ+ Identity and Wellbeing
- Australia’s Clinical Practice Guideline for MDMA
- Rising Ketamine Addiction in the UK
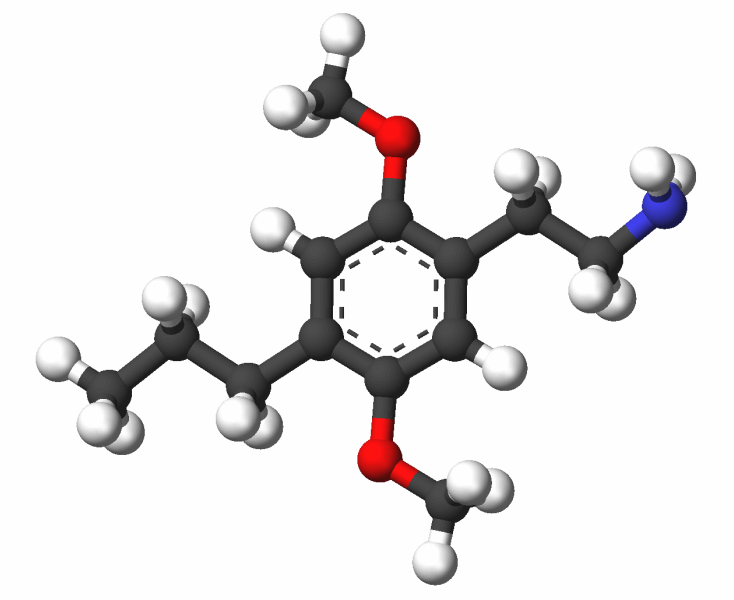
- The Brain on Psychedelics: Psilocybin vs Ketamine
- Final Thoughts
7 minute read
0
Last updated August 11th, 2025










































share your toughts
Join the Conversation.