Stroke, traumatic brain injury, and neurodegenerative diseases together constitute major causes of disability worldwide, exerting a huge personal and economic toll, with a notable lack of effective treatment options for all of these conditions.
Among non-communicable (or transmissible) disorders, stroke is the second leading cause of death and the third leading cause of death and disability combined in the world. Up to half of all stroke survivors will experience long-term disability, and strokes impose a vast personal and economic toll. It is thought that if current trends continue, by 2030, the cost of stroke to the global economy will be over US$1 trillion.
Neurodegenerative diseases such as dementia, Alzheimer’s disease, and Parkinson’s disease impose a vast and growing issue, with dementia alone costing the global economy over $1 trillion a year. Its burden is only projected to increase, with a widescale demographic shift toward an ageing population across much of the world.
Traumatic brain injury (TBI), including concussions, impacts nearly 69 million people worldwide each year, with existing treatments being scarce. It is a multifaceted condition that can cause short- and long-term impairments in physical, cognitive, emotional, and psychological functioning. Psychological impairments may include depression, anxiety, PTSD, irritability, loss of pleasure, chronic pain, and increased suicidality. Certain populations, such as those in military service, emergency first responders and athletes who play contact sports, may be at particular risk of TBI.
TBI also significantly accelerates brain ageing, causing structural changes that make the brain appear older than a person’s chronological age, a gap that often widens over time. In addition to being associated with short-term issues with cognition, behaviour and motor skills, mild, repetitive sub-concussive head injury is a known risk factor for neurodegenerative disease, including dementia, Parkinson’s disease and chronic traumatic encephalopathy, costing billions of dollars in health care.




















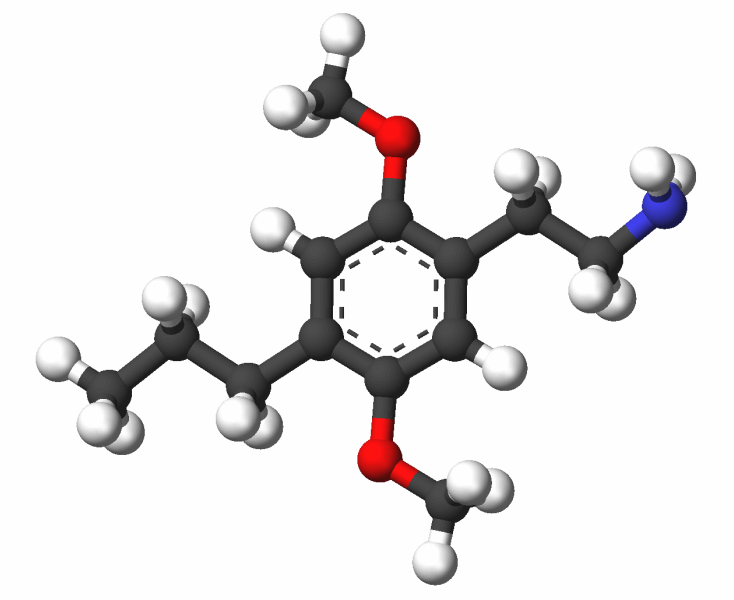























share your toughts
Join the Conversation.